Product Usage: This product is intended solely for use as a research chemical. It is designated exclusively for in vitro research and laboratory testing. All information provided on this site is for educational purposes only. It is strictly prohibited to administer this product to humans or animals. Only licensed and qualified professionals should handle it. This product is not classified as a drug, food, or cosmetic and must not be misrepresented or used as such. This product is for research use only. Not for human consumption.
Tesamorelin
Tesamorelin is a synthetic peptide made up of 44 amino acids and functions as an analogue of growth hormone–releasing hormone (GHRH). It has been studied primarily for addressing abnormal fat accumulation associated with certain chronic conditions, where it has demonstrated a notable ability to reduce deep abdominal fat stores. Investigations have also explored its potential to support peripheral nerve health and to slow the development of early cognitive decline. In specific research groups, tesamorelin has been shown to cut body fat levels by close to one-fifth, with findings indicating it may outperform many other available approaches to reducing excess adiposity in these cases.
Price range: $110.00 through $170.00
Cellular Age Support Peptides
Cellular Growth Research Peptides
Metabolic Activation Peptides
Metabolic Research Peptides
Popular Peptides
Reproductive System Research Peptides
Tissue Integrity Research Peptides
Uncategorized
- DESCRIPTION
- STORAGE
What Is Tesamorelin?
Tesamorelin is a growth hormone–releasing hormone (GHRH) analogue consisting of standard GHRH with an added trans-3-hexanoic acid group. Tesamorelin was approved by the FDA in 2010 for the treatment of HIV-associated lipodystrophy. It has also been investigated for its potential to support peripheral nerve regeneration and as a possible intervention for mild cognitive impairment (MCI), a clinical precursor to dementia.
Tesamorelin Structure
Sequence (Single Letter): Unk-Tyr-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-Gln-Gln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu
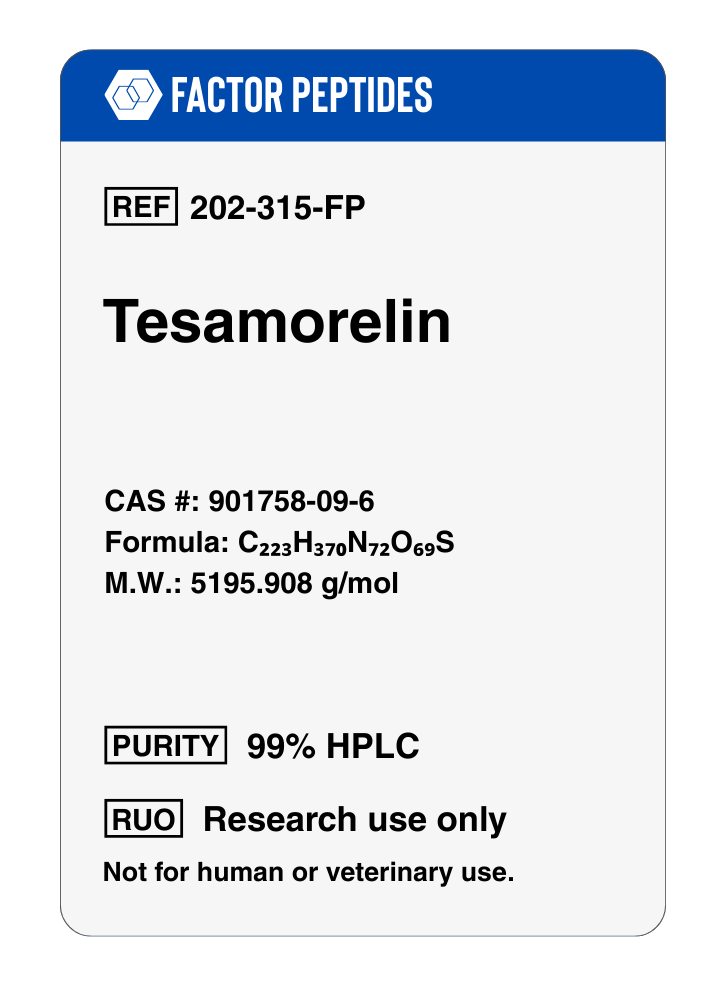
Molecular Formula: C223H370N72O69S
Molecular Weight: 5195.908 g/mol
PubChem CID: 44147413
CAS Number: 901758-09-6
Tesamorelin Research
As a GHRH analogue, tesamorelin shares the core biological actions of GHRH and related analogues such as sermorelin, GRF (1–29), and CJC-1295. The addition of the trans-3-hexanoic acid group improves tesamorelin’s stability in human plasma and prolongs its half-life. Despite this increased stability, tesamorelin, like CJC-1295, maintains the physiological pulsatile pattern of growth hormone (GH) release, which is associated with a lower rate of side effects compared to agents that disrupt normal GH pulsatility.
Tesamorelin and Lipodystrophy
The primary approved use for tesamorelin is in the treatment of HIV-associated lipodystrophy, a condition that arises both as a consequence of HIV infection and as a side effect of antiretroviral therapy. Lipodystrophy is characterized by abnormal fat accumulation, particularly in the abdomen and in certain peripheral regions. Although the exact mechanisms are not fully defined, widely used protease inhibitors are thought to contribute significantly to the development of this condition.
Historically, patients with lipodystrophy had limited options: lifestyle modification through diet and exercise, along with a small number of largely ineffective medications. When these measures failed, surgery was sometimes used as a last resort, often with mixed results and significant risk. The approval of tesamorelin in 2010 provided a targeted pharmacologic option. In clinical studies, tesamorelin has been shown to reduce visceral adiposity by nearly 20% in HIV-associated lipodystrophy. Research suggests that tesamorelin is roughly four times more effective in reducing adiposity than all previously available therapies combined.
Tesamorelin Investigated in Cardiac Disease
Individuals living with HIV have an elevated risk of cardiovascular disease (CVD), due both to altered fat distribution and to the metabolic effects of antiretroviral drugs. After effective viral suppression with highly active antiretroviral therapy (HAART), prevention of CVD is considered one of the most important priorities in long-term care. Until recently, statins and lifestyle measures formed the backbone of risk reduction in this population.
Clinical research indicates that tesamorelin not only decreases visceral adiposity, but also reduces triglyceride levels, total cholesterol, and non–HDL cholesterol in HIV-positive patients. A 15% reduction in visceral adipose tissue induced by tesamorelin has been associated with an average decrease of approximately 50 mg/dL in triglyceride levels.
Because ectopic fat deposition is linked to chronic inflammation, and inflammation is a well-recognized risk factor for CVD, reducing depots such as visceral abdominal fat, liver fat, and epicardial fat may help lower overall cardiovascular risk. By lowering these ectopic fat stores, tesamorelin may indirectly attenuate inflammation and support improved cardiometabolic health in HIV-positive individuals.
Growth Hormone Deficiency and HIV
Emerging evidence suggests that HAART is associated with endocrine and metabolic disturbances, including a higher incidence of growth hormone deficiency. HIV infection itself, along with its treatment, appears to affect pituitary function. As a result, roughly one third of HIV-positive patients receiving HAART may have suboptimal GH production.
This observation likely helps to explain both the high prevalence of lipodystrophy and the robust response to tesamorelin in this population. By stimulating endogenous GH release through the GHRH pathway rather than providing exogenous GH directly, tesamorelin offers a more physiological and often safer means of increasing GH levels, particularly in individuals with complex comorbidities such as HIV.
Tesamorelin for Peripheral Nerve Damage
Peripheral nerve damage can occur due to trauma, diabetes, toxic exposures, or surgical procedures, often resulting in persistent motor and sensory impairment. Because peripheral nerves regenerate slowly and incompletely, current treatment options are limited. Experimental work has suggested that manipulating GH signaling may enhance nerve regeneration, support remyelination, and improve functional recovery.
Tesamorelin, thanks to its established safety profile and FDA approval for lipodystrophy, has become a leading candidate among GHRH analogues for exploring strategies to improve peripheral nerve healing in clinical and translational research.
Tesamorelin Investigated in Dementia
There is growing interest in the potential cognitive effects of GHRH analogues such as tesamorelin. A large, randomized, double-blind, placebo-controlled study conducted at the University of Washington School of Medicine over a 20-week period reported that GHRH analogues can improve cognitive function in individuals with mild cognitive impairment (MCI) and in some older adults without dementia.
In these studies, tesamorelin and related molecules are thought to act, in part, by increasing gamma-aminobutyric acid (GABA) levels and decreasing myo-inositol (MI) levels in specific brain regions, changes that may reflect improved neuronal function and reduced glial activation. These findings not only support continued investigation of tesamorelin as a possible intervention in early cognitive decline, but also point to new biochemical pathways that may be relevant for the prevention and treatment of neurodegenerative disease.
Tesamorelin Research
Because tesamorelin is already FDA approved for use in humans, it represents an attractive option for ongoing and future clinical research. Current and planned studies are evaluating its ability to reduce cardiovascular risk in HIV, support peripheral nerve repair after injury, and slow or modify the progression of cognitive decline and dementia. Multiple clinical trials are underway across these different areas.
Tesamorelin exhibits minimal side effects and demonstrates low oral and excellent subcutaneous bioavailability in mice. Dose levels used in animal models do not scale directly to humans. Tesamorelin for sale at Peptide Sciences is supplied strictly for educational and scientific research purposes only, and is not intended for human consumption or therapeutic use. Only licensed and qualified researchers should purchase Tesamorelin.
Factor Peptides Storage Guidelines:
These peptides arrive in a dried, stabilized form produced by a process called lyophilization, or freeze drying. In this state, they are generally suitable for transport and short-term storage at typical room temperatures for several months.
Once the dry powder is mixed with bacteriostatic water and turned into a liquid solution, the storage requirements change. The reconstituted solution should be kept in a refrigerator to help maintain its properties, and is usually considered suitable for use for about 30 days under chilled conditions.
Lyophilization involves freezing the material and then exposing it to low pressure so that ice in the sample passes directly from solid to vapor, rather than melting. This leaves behind a light, porous, white solid that is more stable than the original liquid. In this form, the product can often be kept at room temperature until it is time to add diluent.
After delivery, it is good practice to protect the vials from heat and strong light. If the product will be used in the near future, storing the lyophilized powder or reconstituted solution in a refrigerator at temperatures below about 4°C (39°F) is typically appropriate. The dry form often remains intact at room temperature for a number of weeks, so this may also be acceptable when immediate refrigeration is not available and the intended use is relatively soon.
For storage over longer periods, such as many months or years, much colder conditions are preferred. Placing the vials in a deep freezer, around -80°C (-112°F), is commonly used to help preserve the structure and activity of peptides for extended time frames.