Product Usage: This product is intended solely for use as a research chemical. It is designated exclusively for in vitro research and laboratory testing. All information provided on this site is for educational purposes only. It is strictly prohibited to administer this product to humans or animals. Only licensed and qualified professionals should handle it. This product is not classified as a drug, food, or cosmetic and must not be misrepresented or used as such. This product is for research use only. Not for human consumption.
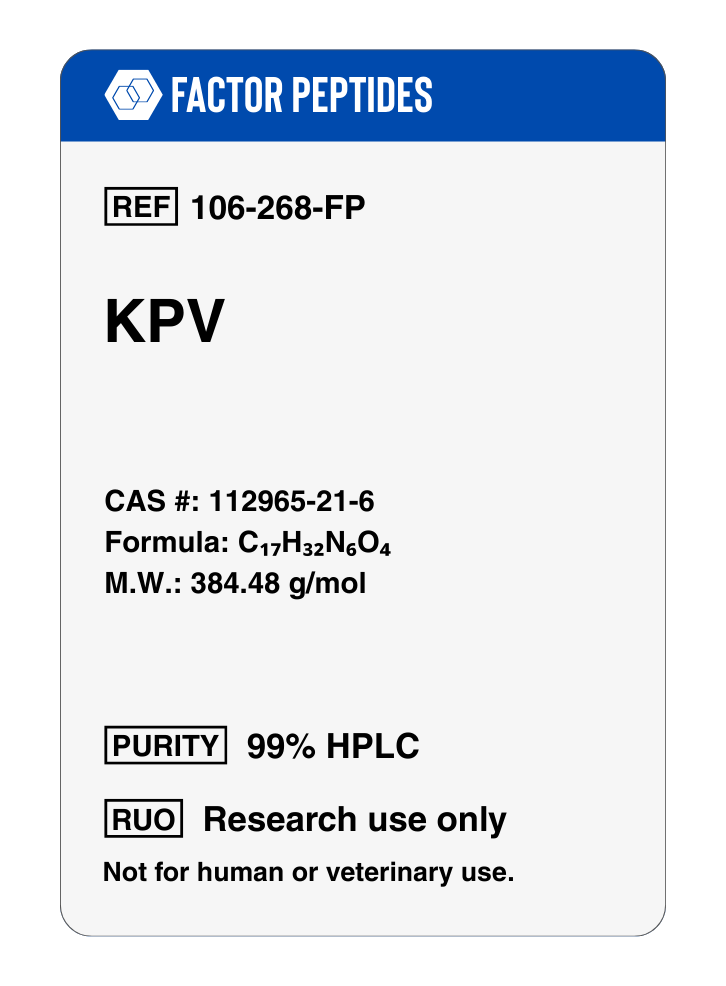
KPV
KPV (Ac-KPV-NH2) is a short peptide with strong inflammation-modulating effects that is being studied across several health-related applications. A major focus of current research is its use in inflammatory bowel conditions, where early findings have been highly encouraging. Studies on tissue repair also suggest that Ac-KPV-NH2 and other related fragments may help wounds close more quickly, lower the chance of infection, calm local inflammation, and support more favorable long-term skin appearance. Because of these combined actions, Ac-KPV-NH2 and similar peptides are being explored as potential key tools in both wound care and in minimizing scarring after surgical procedures.
Price range: $40.00 through $55.00
All Products
- DESCRIPTION
- STORAGE
Ac-KPV-NH2 Overview
Ac-KPV-NH2 is a short peptide fragment derived from a larger melanocortin hormone. It is one of several minimal sequences created to see whether small pieces of the original molecule could preserve useful biological actions such as protection against light damage, support in low-oxygen conditions, and influence over appetite and energy balance. Composed of just three amino acids, it has drawn attention primarily for its strong anti-inflammatory activity. Experimental work suggests that it can calm excessive immune reactions in many tissues, including the digestive tract, brain, lungs, blood vessels, and joints. Because of its small size, it is being evaluated in multiple delivery formats, including oral, injectable, and skin-based preparations.
Ac-KPV-NH2 Research
Intestinal Inflammation
One of the most striking findings about Ac-KPV-NH2 is its ability to dampen inflammation in the intestines. In animal models that mimic inflammatory bowel disease, treatment with this peptide has been associated with less inflammatory cell infiltration, lower markers of tissue damage, and improved microscopic appearance of the gut lining. Animals receiving the peptide often regain weight and recover more quickly than untreated controls, suggesting a meaningful impact on disease severity.
Investigators have also explored ways to steer Ac-KPV-NH2 more precisely to inflamed areas of the intestine. One approach has been to attach the peptide to specialized particles coated with substances that guide them toward damaged gut tissue. This targeted system appears to speed up repair of the intestinal surface and reduce inflammation, in part by lowering levels of key inflammatory signaling proteins. The primary advantage of these delivery strategies is improved oral performance, allowing more of the peptide to reach its intended site of action while maintaining the same basic mechanism.
Another important observation is that Ac-KPV-NH2 seems to act mainly where inflammation is already present. In healthy tissue, it has little measurable impact. This selectivity is linked to transport pathways in the intestinal wall that become more active during inflammatory flares, allowing greater uptake of the peptide into affected cells. As a result, Ac-KPV-NH2 is being considered as a potential long-term maintenance option for chronic intestinal conditions. The idea is that it could be taken regularly, remain largely inactive in calm periods, and become more effective during flares when the relevant transport systems are upregulated. This concept of using disease-specific transport or signaling changes to concentrate drug action in problem areas may also be applicable to other conditions beyond the gut.
Ac-KPV-NH2 as a General Anti-Inflammatory
Work dating back several decades showed that small fragments related to melanocortin hormones can reduce both fever and inflammation in animal models. Ac-KPV-NH2 proved to be a strong anti-inflammatory agent in these early experiments, although the complete parent hormone generally produced the most powerful effect on body temperature. These findings prompted a long line of research into different truncated and modified variants to see which parts of the molecule are essential for various actions.
Across many studies, the broader melanocortin family has consistently demonstrated anti-inflammatory activity in a wide range of conditions, including fever, irritated or allergic skin reactions, blood vessel inflammation, tissue scarring, joint inflammation, and inflammatory responses in organs such as the eyes, lungs, brain, and digestive system. While the full-length hormone typically exhibits the greatest potency, it also darkens the skin, a property that limits its practical use. Ac-KPV-NH2, in contrast, keeps most of the desired anti-inflammatory actions without altering skin color, making it more attractive for long-term or higher-dose use when needed.
The difference in strength between the full-length hormone and Ac-KPV-NH2 is often modest, especially in the early stages of an inflammatory reaction where the smaller peptide appears to account for much of the benefit. Where the parent hormone seems to stand out is in long-term modulation of immune responses. In some models of allergic skin irritation, for example, it provides greater protection against delayed reactions that appear weeks after the initial trigger. This suggests that the larger molecule may be engaging additional immune-regulating pathways that the smaller fragment does not fully influence, an area that continues to be actively studied.
Wound Healing
Wound repair is a staged process that progresses through inflammatory, proliferative, and remodeling phases, each with its own mix of cell types and signaling molecules. Research has shown that many of the skin cells involved at different stages carry receptors that recognize melanocortin hormones and their smaller analogues. This means that both the full hormone and shortened fragments such as Ac-KPV-NH2 can directly influence cells responsible for closing wounds and rebuilding tissue.
Derivatives like Ac-KPV-NH2 are particularly interesting because they preserve important inflammation-calming and protective properties of the parent hormone but lack its pigmenting effect. This combination makes them appealing candidates for supporting tissue repair while minimizing changes in skin color or the exaggerated scar patterns that can occur in some individuals, especially those with naturally darker complexions.
Ac-KPV-NH2 also appears to play a role in the body’s first-line defense against common skin microbes. Experimental data indicate that it can inhibit the growth of certain bacteria and fungi at concentrations believed to be achievable in living tissue. This dual action, both reducing inflammation and limiting pathogen growth, suggests possible advantages in situations such as burns or large injuries where infection risk is high. Unlike some conventional anti-inflammatory treatments that may dampen immune defenses, this peptide has the potential to reduce damaging inflammation while still supporting antimicrobial protection.
Because of its antifungal characteristics and defined three-dimensional shape, Ac-KPV-NH2 has served as a template for designing new compounds aimed at controlling fungal infections. Researchers are exploring how to mimic the structural features responsible for its antimicrobial activity while fine-tuning other aspects of function for specialized medical applications.
Scar Formation
Given its impact on early inflammation, scientists have also evaluated Ac-KPV-NH2 during later stages of wound repair, when the body is rebuilding tissue and shaping the final scar. Persistent or excessive inflammation during this time can lead to raised, thick, or spreading scars. Studies suggest that treatment with melanocortin-based peptides can reduce the intensity of chronic inflammatory changes associated with aggressive scar formation, leading to smaller and less prominent scars. Similar patterns have been observed in other organs, such as the lungs and heart, where these molecules appear to soften or limit fibrotic changes.
Part of this effect seems tied to how these peptides influence collagen turnover and the signaling molecules that regulate it. They have been shown to decrease certain inflammatory messengers that drive excessive production of a key collagen subtype. This is especially relevant in the final remodeling phase of healing, since individuals prone to keloids or hypertrophic scars often show differences in receptor expression on the cells that create connective tissue. By moderating this signaling environment, Ac-KPV-NH2 may help keep collagen deposition closer to normal, resulting in smoother, less conspicuous scar tissue.
Ac-KPV-NH2 Compared with Its Parent Hormone
When Ac-KPV-NH2 is compared to the original melanocortin hormone from which it is derived, a clear trade-off emerges. The full-length molecule generally produces stronger anti-inflammatory effects but also reliably increases skin pigmentation, an outcome that many patients and clinicians would find undesirable outside of specific cosmetic contexts. Ac-KPV-NH2, on the other hand, maintains most of the beneficial inflammation-limiting properties while avoiding this major drawback. Its compact structure also makes it relatively inexpensive and practical to manufacture, which is helpful for large-scale study and potential future use.
Interestingly, the small fragment does not appear to rely on the same receptor mechanisms as the parent hormone for its anti-inflammatory actions. Experiments in which the main receptors for the full-length molecule are deliberately blocked show that these interventions do not eliminate the effects of Ac-KPV-NH2 on immune cell movement and inflammatory signaling. This indicates that it is acting through distinct pathways, which may offer new opportunities for targeting inflammation without engaging all of the effects associated with the larger hormone.
Another advantage of Ac-KPV-NH2 is its flexibility in how it can be given in experimental settings. Animal studies have successfully used oral dosing, injections beneath the skin, direct injections into body cavities or the central nervous system, and topical or transdermal approaches. Each route influences how quickly and where in the body the peptide exerts its effects, allowing researchers to tailor delivery toward particular tissues or disease processes.
Ac-KPV-NH2 Summary
Overall, Ac-KPV-NH2 is a compact, strongly anti-inflammatory peptide with promising activity across several experimental disease models. It has shown particular potential in conditions involving intestinal inflammation, where it can lessen disease severity and promote healing of the gut lining, and in wound care, where it may help speed closure, limit infection, and improve the appearance of scars. Its ability to act through multiple routes of administration further enhances its appeal as a versatile research tool.
Current evidence comes primarily from laboratory and animal studies, which also highlight that the way this peptide behaves in small animals cannot be directly translated to people without careful testing. Early work suggests that it has a favorable safety profile, with especially good performance when given by injection and more limited activity by mouth unless special delivery methods are used. At present, Ac-KPV-NH2 is being explored for scientific and educational purposes and is not approved for use in humans.
Factor Peptides Storage Guidelines:
These peptides arrive in a dried, stabilized form produced by a process called lyophilization, or freeze drying. In this state, they are generally suitable for transport and short-term storage at typical room temperatures for several months.
Once the dry powder is mixed with bacteriostatic water and turned into a liquid solution, the storage requirements change. The reconstituted solution should be kept in a refrigerator to help maintain its properties, and is usually considered suitable for use for about 30 days under chilled conditions.
Lyophilization involves freezing the material and then exposing it to low pressure so that ice in the sample passes directly from solid to vapor, rather than melting. This leaves behind a light, porous, white solid that is more stable than the original liquid. In this form, the product can often be kept at room temperature until it is time to add diluent.
After delivery, it is good practice to protect the vials from heat and strong light. If the product will be used in the near future, storing the lyophilized powder or reconstituted solution in a refrigerator at temperatures below about 4°C (39°F) is typically appropriate. The dry form often remains intact at room temperature for a number of weeks, so this may also be acceptable when immediate refrigeration is not available and the intended use is relatively soon.
For storage over longer periods, such as many months or years, much colder conditions are preferred. Placing the vials in a deep freezer, around -80°C (-112°F), is commonly used to help preserve the structure and activity of peptides for extended time frames.